Fluoride Compound Poisonous to Humans and Livestock
 | ||
| Exposure to Fluorine |
Fluorides are highly toxic to humans and livestock. I was shocked that someone at the CDC would pretend
otherwise. Whomever wrote the fact sheet should probably lose their job over such a patently false and misleading statement. Fluorides in fact leads to the degradation of teeth and bones, mental instability, neurological damage, i.q. damage and lowering, lowered sperm count, infertility, brittle bones, skin irritation, eye irritation, respiratory tract chronic disorders, heart disorders and damage, cancer and of course death.
I don't know who in your organization is putting forth false claims of fluoride as being any benefit to humans.
Quite frankly it is an industrial waste product and I'm sure the clean up is quite expensive. But, apparently you need to look out for nefarious individuals in your organization trying to fool the general populous. It is unconscionable to think someone would lead civilians astray to the point that they could expose themselves to fluoride, ultimately resulting in death. It would be on par of someone saying mercury was a cure for skin rashes or stomach ache. It is just unthinkable someone would say that.
Respectfully,
Very concerned citizen
P.S. I would hope you would do something to remedy
this very bad and false information. People could be dying from exposure
to fluoride.
That was a letter sent to the CDC directly. A response has not been forthcoming.& But just so everyone is crystal clear on what Fluorine is, here is the real information on it. Just because you see a commercial advertising something, does not make it truth, nor real.
Fluorine is the chemical element with atomic number 9, represented by the symbol F. Fluorine forms a single bond with itself in elemental form, resulting in the diatomic F2 molecule. F2 is a supremely reactive, poisonous, pale, yellowish brown gas. Elemental fluorine is the most chemically reactive and electronegative of all the elements. For example, it will readily "burn" hydrocarbons at room temperature, in contrast to the combustion of hydrocarbons by oxygen, which requires an input of energy with a spark. Therefore, molecular fluorine is highly dangerous, more so than other halogens such as the poisonous chlorine gas.
Fluorine's highest electronegativity and small atomic radius give unique properties to many of its compounds. For example, the enrichment of 235U, the principal nuclear fuel, relies on the volatility of UF6. Also, the carbon–fluorine bond is one of the strongest bonds in organic chemistry. This contributes to the stability and persistence of fluoroalkane based organofluorine compounds, such as PTFE/(Teflon) and PFOS. The carbon–fluorine bond's inductive effects result in the strength of many fluorinated acids, such as triflic acid and trifluoroacetic acid. Drugs are often fluorinated at biologically reactive positions, to prevent their metabolism and prolong their half-lives.
Fluorine salts, known as fluorides, were used for centuries in welding metals and for frosting glass before the element itself was isolated. Fluorine gas is the most reactive of all the elements and quickly attacks all metals - steel wool bursts into flames when exposed to it! Fluorine is used to make uranium hexafluoride, needed by the nuclear power industry, and sulfur hexafluoride insulating gas for high-power electricity transformers, and to treat polythene to make it impermeable to solvents.
Fluoride causes dental fluorosis, a condition that makes teeth hard and brittle with discoloration, chipping and pitting of the enamel. It's estimated by the Centers for Disease Control that one-third of the children in the United States have dental fluorosis. In Canada the numbers are lower due to less fluoride in the water: Health Canada estimates between 12 and 14 percent incidence of fluorosis. Furthermore new studies show that the supposed benefits of fluoridation are nonexistent. The National Institute of Dental Research conducted the largest study to date on fluoride's effects on teeth with over 39,000 children ages 5 to 17 and found no significant differences between fluoridated and non-fluoridated communities. Another study in New York State found that the only significant difference is that fluoridated Newburgh, New York, has twice the dental fluorosis of non-fluoridated Kingston, New York.
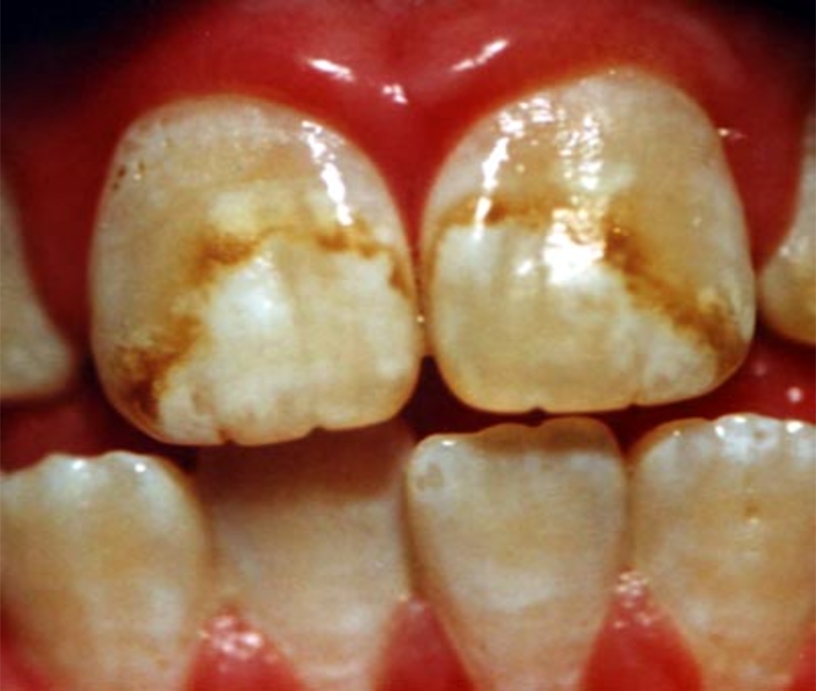 |
| Dental Fluorosis |
That same project went and got "doctors" to declare that fluoride was safe for human consumption and prevents tooth decay. So in one fell swoop they got all their lawsuits tossed out and they no longer had to dump fluoride, they could put it in water, and toothpaste. So not only did they stop having to pay rather expensive disposal of fluoride, they actually made money off of the waste product.
Any chemist worth his salt, and who is not on the take, will readily tell you that fluoride is a waste product and not suitable for human nor livestock consumption.
Please visit my legal website: DUI
See me on YouTube: Shakaama Live
Need a Notary in Las Vegas Nevada Notary Public Nevada

6 comments:
Shocking. What's the other side of the story? I mean the government has to say something to support all they are doing. What's that? We'd like to read a balanced exposure of all facts.
Thanks.
- Prof U.K. Bhadra
Balance? You want to hear the government side of the story? This IS the other side of the story. You've never heard this side. You've only heard the government's side of the story.
It is amazing how people come to my blog and all of a sudden try to appear fair, impartial and logical about topics. When the fact of the matter is, the media, advertising and politicos have spun only one side of the story for decades, if not a century or more on most topics.
Thanks for the laugh buddy.
Do you Know Paul Connett or Daniel Stockin?Are you a lawyer?
You would be a little more convincing if you mentioned the fact that fluoride is poisonous in this way if taken in large doses. Small doses of around 2.5mg per day have been proven in studies to be very beneficial to skeletal structures and tooth enamel.
Clearly you are convinced of the opposite, that fluoride is some how beneficial.
Until I point out the fact that fluoride that you think is in your toothpaste is not fluoride at all. It is:
"The chemicals - fluorosilicic acid, sodium silicofluoride, and sodium fluoride - used to fluoridate drinking water are industrial waste products from the phosphate fertilizer industry. Of these chemicals, fluorosilicic acid (FSA) is the most widely used. FSA is a corrosive acid which has been linked to higher blood lead levels in children. A recent study from the University of North Carolina found that FSA can - in combination with chlorinated compounds - leach lead from brass joints in water pipes, while a recent study from the University of Maryland suggests that the effect of fluoridation chemicals on blood lead levels may be greatest in houses built prior to 1946. Lead is a neurotoxin that can cause learning disabilities and behavioral problems in children."
So your comment is null and void and purely propaganda put out by the waste management industry to get rid of industrial waste. 97% of all European countries ban and outlaw any dumping of toxic fluorides into the drinking water.
As a chemist - (MChem, Hons., Southampton, UK) - the biological activity of a given compound depends entirely on its particular form ie. F(ABC) could be deadly, whilst F(BCD) can form an essential nutrient. Painting Flourine as black or white is naive. Flouridated toothpastes/water supplies for this reason do not contain Flourine gas, for example.
Post a Comment